Polarimetry Principle Instrumentation and Applications
Polarimetry in Pharmaceutical Analysis: Principle Instrumentation, and Applications
Introduction
In the pharmaceutical industry, the quality and purity of drugs are of utmost importance. Many medicines and natural products are optically active, meaning they can rotate the plane of polarized light. The scientific technique used to study this property is known as polarimetry.
Polarimetry is simple, rapid, and non-destructive, making it highly valuable for analyzing chiral drugs, natural products, and sugar-containing formulations. By measuring the angle of rotation, pharmacists and analysts can identify, quantify, and check the purity of pharmaceutical substances.
In this article, we will discuss the principle, instrumentation, and major applications of polarimetry in drug analysis, highlighting its role in ensuring the safety and efficacy of medicines.
Principle of Polarimetry
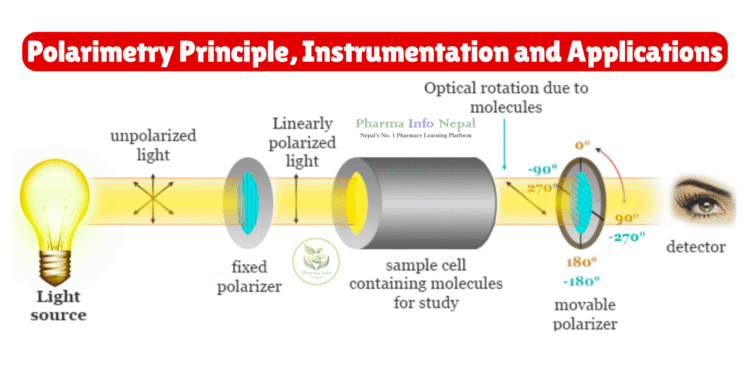
Polarimetry is based on the ability of optically active substances (chiral compounds) to rotate the plane of polarized light.
- When plane-polarized light passes through an optically active compound, the orientation of the light is rotated either to the right (dextrorotatory, +) or to the left (levorotatory, –).
- The angle of rotation (α) depends on:
- Nature of the compound (its optical activity)
- Concentration of the solution
- Path length of the light through the solution
- Wavelength of light used (commonly sodium D-line, λ = 589 nm)
- Temperature

Instrumentation of Polarimetry
A typical polarimeter consists of the following parts:
- Light Source
- Monochromatic light is required.
- Commonly sodium vapor lamp (589 nm, D-line).
- In modern instruments, LED with filters is also used.
- Polarizer
- Nicol prism or polarizing filter is used to produce plane-polarized light.
- Sample Tube (Polarimeter Tube)
- A transparent cylindrical tube, usually 1–10 dm in length.
- Filled with the optically active drug solution.
- Analyzer
- Another Nicol prism or polarizer placed after the sample.
- Detects the change in the plane of polarization.
- Detector & Scale
- Detects the rotated light.
- The angle of rotation is measured directly on a circular scale or digitally displayed.
- Temperature Control (optional)
- Since optical rotation depends on temperature, some instruments include thermostatic control.
Applications of Polarimetry in Drug Analysis
Polarimetry is widely used in pharmaceutical quality control and analysis, especially for chiral drugs and natural products.
1. Identification of Drugs
- Many natural drugs (alkaloids, glycosides, terpenes, essential oils) are optically active.
- Example: Quinine, morphine, menthol, camphor.
2. Purity Testing
- Impurities or racemization reduce specific rotation.
- Used for testing raw materials like menthol, camphor, and essential oils.
3. Quantitative Estimation
- Based on Biot’s law (specific rotation formula), concentration of optically active drugs can be determined.
- Example: Determination of glucose concentration in IV fluids, sucrose in syrups.
4. Distinction Between Isomers
- Enantiomers rotate light in opposite directions (+ or –).
- Example: (+)-ephedrine vs (–)-ephedrine.
- Polarimetry helps in studying chirality and stereochemistry of drugs.
5. Quality Control in Industry
- Used to check the authenticity of essential oils (peppermint oil, spearmint oil, etc.).
- Applied in sugar industry and fermentation industry as well.
Summary:
Polarimetry is a simple, non-destructive, and reliable method to study optical activity. In pharmaceuticals, it is important for identification, purity testing, concentration determination, and stereoisomer differentiation of optically active drugs.
- UV Spectrophotometry: Principle, Instrumentation and Applications
- Infrared (IR) Spectroscopy: Principle, Instrumentation, and Applications
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Principle, Instrumentation, and Applications